
D-Dimer Testing Market Set to Reach $2.2 Billion by 2032, Growing at 5.1% CAGR
D-dimer Testing Market
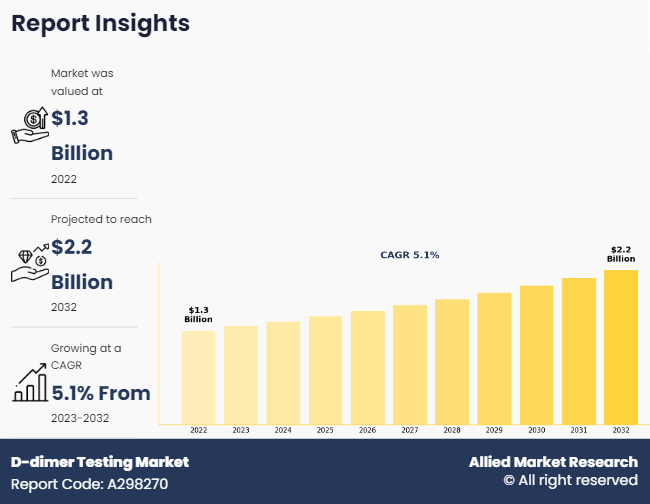
The D-dimer testing market was valued at $1.3 billion in 2022 and is projected to grow to $2.2 billion by 2032, at a CAGR of 5.1%.
WILMINGTON, DE, UNITED STATES, November 30, 2024 /EINPresswire.com/ -- The D-dimer testing market is an integral part of modern diagnostics, addressing conditions linked to blood clot formation. According to Allied Market Research, the D-dimer testing market was valued at $1.3 billion in 2022 and is projected to grow to $2.2 billion by 2032, at a CAGR of 5.1%. The steady growth reflects advancements in diagnostic technologies, increasing awareness of thrombotic conditions, and rising global healthcare needs.
Get Sample PDF Copy of Report: https://www.alliedmarketresearch.com/request-sample/A298270
What is D-dimer Testing?
D-dimer testing is a diagnostic procedure used to detect fibrin degradation products, which are released when blood clots dissolve. Elevated levels of D-dimer indicate potential clotting disorders such as:
Deep vein thrombosis (DVT)
Pulmonary embolism (PE)
Disseminated intravascular coagulation (DIC)
While highly sensitive, the test is not specific enough to confirm diagnoses independently, often necessitating supplementary imaging or clinical evaluations.
D-dimer Testing Market Drivers
1. Rising Prevalence of Thrombotic and Cardiovascular Disorders
Thrombosis and cardiovascular diseases are leading causes of morbidity and mortality worldwide. The ability of D-dimer tests to detect early clot formation makes them indispensable in:
Diagnosing venous thromboembolism (VTE)
Monitoring myocardial infarction risks
Managing post-surgical complications
Increasing integration of D-dimer testing into diagnostic protocols is driving demand for these tests across healthcare facilities globally.
2. Shift Toward Point-of-Care Testing (POCT)
Traditional D-dimer testing relied heavily on centralized laboratories, which often delayed results. The advent of point-of-care testing (POCT) has transformed this landscape by offering rapid and accurate results, particularly in emergency and critical care settings.
POCT devices reduce diagnostic delays, enabling timely intervention in life-threatening conditions like PE or DVT.
This shift aligns with the global emphasis on decentralized healthcare delivery and patient-centric care.
3. Advancements in Diagnostic Technologies
The ongoing development of automated testing systems and high-sensitivity assays has improved the reliability and efficiency of D-dimer testing.
Companies like Thermo Fisher Scientific and Siemens Healthineers are investing in next-generation technologies to enhance diagnostic precision.
Integration of AI-based diagnostic tools is expected to further optimize test interpretation and clinical decision-making.
Challenges in the D-dimer Testing Market
1. False Positives and Diagnostic Limitations
While D-dimer tests are valuable for ruling out clotting disorders, elevated levels can also result from non-thrombotic conditions, including:
Inflammation
Infections
Post-surgical recovery
This lack of specificity complicates diagnosis, requiring clinicians to combine test results with patient history, physical examination, and imaging studies.
2. Cost Constraints and Limited Access
In some regions, the cost of advanced D-dimer testing and limited access to diagnostic infrastructure remain barriers. Efforts to expand affordable testing options are crucial for broader market penetration.
3. Impact of Regulatory Standards
Strict regulations govern the production, use, and interpretation of diagnostic tests. Manufacturers must navigate complex compliance frameworks, which can slow product development and market entry.
D-dimer Testing Market Segmentation
By Test Type: Laboratory Tests and Point-of-Care Tests
Laboratory tests currently dominate the market, providing detailed and accurate results for complex cases.
The point-of-care testing segment is expected to grow rapidly, driven by demand for quick and portable diagnostic solutions.
By Application: DVT, PE, DIC, and Others
Deep vein thrombosis (DVT) accounted for the largest market share in 2022, as early detection can significantly reduce mortality.
Pulmonary embolism (PE) is anticipated to grow at the fastest rate, reflecting rising awareness of this life-threatening condition.
By End Use: Hospitals, Diagnostic Centers, and Research Institutes
Hospitals remain the largest end-users, leveraging D-dimer tests in emergency and critical care.
Diagnostic laboratories and research institutes are also significant contributors, focusing on refining test accuracy and expanding clinical applications.
Have Any Query? Ask Our Expert: https://www.alliedmarketresearch.com/purchase-enquiry/A298270
By Region: North America, Europe, Asia-Pacific, and LAMEA
North America led the market in 2022, driven by advanced healthcare infrastructure, high disease prevalence, and technological innovation.
Asia-Pacific is expected to witness the fastest growth, with increasing investments in healthcare and rising awareness about thrombotic disorders.
D-dimer Testing Market Competitive Landscape
Key players driving the D-dimer testing market include:
Thermo Fisher Scientific Inc.
F. Hoffmann-La Roche Ltd.
Siemens Healthineers
Abbott
bioMérieux SA
These companies are focusing on R&D, product launches, and strategic partnerships to strengthen their market positions.
D-dimer Testing Market Future Trends and Opportunities
1. Expanding Research on D-dimer Levels
Ongoing studies are uncovering new clinical correlations between D-dimer levels and various health conditions, such as:
COVID-19 complications
Stroke risk assessment
Cancer-related thrombosis
This research is broadening the scope of D-dimer testing across diverse medical specialties.
2. Integration of AI and Machine Learning
AI-powered diagnostic tools are enhancing test accuracy and workflow efficiency. These technologies aid in interpreting complex data, reducing diagnostic errors, and improving patient outcomes.
3. Growth in Emerging Markets
Emerging economies in Asia-Pacific, Africa, and Latin America present untapped opportunities. Improving healthcare infrastructure and government initiatives to promote diagnostic awareness are key growth drivers in these regions.
4. Personalized Medicine and Proactive Care
The emphasis on personalized healthcare is driving demand for diagnostic tools like D-dimer tests that enable early intervention and tailored treatment strategies.
The D-dimer testing market is poised for significant growth, driven by rising awareness of thrombotic conditions, technological advancements, and the shift toward point-of-care testing. While challenges like false positives and regulatory hurdles exist, the market’s potential remains robust, supported by ongoing R&D and strategic innovations.
As healthcare systems evolve to prioritize early detection and patient-centric care, D-dimer testing will continue to play a pivotal role in improving diagnostic accuracy and patient outcomes. The next decade promises transformative changes, positioning the D-dimer testing market at the forefront of modern diagnostics.
Procure Complete Report: https://www.alliedmarketresearch.com/d-dimer-testing-market/purchase-options
Thanks for reading this article; AMR also offers Custom Research services providing focused, comprehensive and tailored research according to clientele objectives. Thanks for reading this article; you can also get individual chapter wise sections or region wise reports like North America, Europe, or Asia.
About Us:
Allied Market Research (AMR) is a full-service market research and business-consulting wing of Allied Analytics LLP based in Portland, Oregon. Allied Market Research provides global enterprises as well as medium and small businesses with unmatched quality of "Market Research Reports" and "Business Intelligence Solutions." AMR has a targeted view to provide business insights and consulting to assist its clients to make strategic business decisions and achieve sustainable growth in their respective market domain.
David Correa
Allied Market Research
+1 800-792-5285
email us here
Visit us on social media:
Facebook
X
Distribution channels: Business & Economy, Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release